

Implants



Implants


Loupes


Regeneration


Drills and screws


Surgery


Periodontology


Endodontics
A variety of membranes each with unique resorption time frames and handling characteristics for graft containment applications.

Absorbable collagen membrane

Surgitime seal, mesh, 3d, ptfe i dptfe.
T-Gen is an absorbable dental membrane for guided bone regeneration made of purified collagen without cross-linking or chemical treatment.
Better Handling
Better Stability
Better Tissue Integration

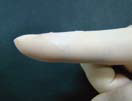
T-Gen has excellent tensile strength and thus, it can enable better handling properties for tear resistance in surgery than other products. In addition, T-Gen features excellent adaptation to surface contour due to fast hydration and excellent flexibility.
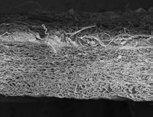
T-Gen has high porosity and specific surface area, which support excellent blood supply. This ensures optimal regeneration of bone and soft tissue.
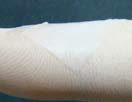
T-Gen functions as a barrier to provide the prolonged space maintenance with the long resorption period. T-Gen is biodegradable and absorbed within 24 weeks after implantation.
| 68 EUR | 15 x 20 | TG-1 |
| 99 EUR | 20 x 30 | TG-2 |
| 167 EUR | 30 x 40 | TG-3 |
Indications: guided tissue regeneration (GTR), guided bone regeneration (GBR)
Sizes: 10x10mm, 15x20mm, 20x30mm, 30x40mm

Composition: original pork pericardium
Smartbrane has adequate tensile strength to safely maintain bone graft stability and structure.

Innovative processing technology that results in a biocompatible membrane. The proprietary cleaning process using supercritical carbon dioxide (scCO2) gently removes unwanted materials (e.g cells, lipids) while preserving the natural collagen matrix and the natural cross-linking of the collagen fibers.
• Porcine pericardium membrane biocompatibility results in adequate barrier functionality.
• Natural collagen matrix plays an important role in blood clotting and enables cell attachment.
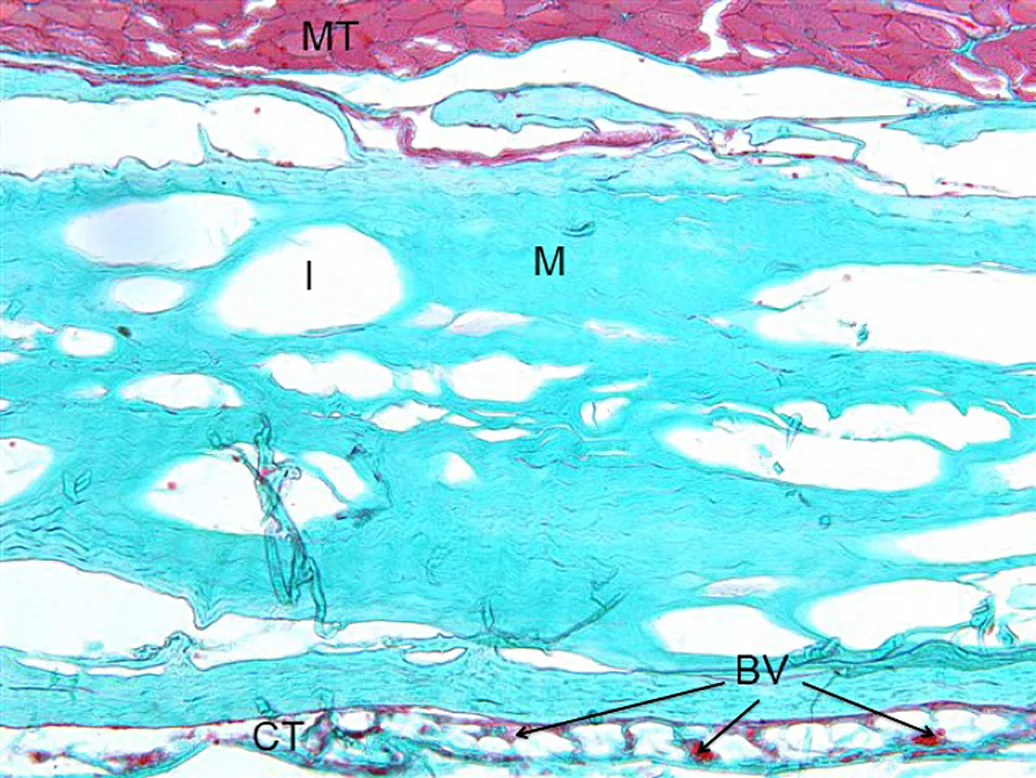
Smartbrane (M) is already connected to the muscular tissue (MT), no signs of inflammatory reactions.
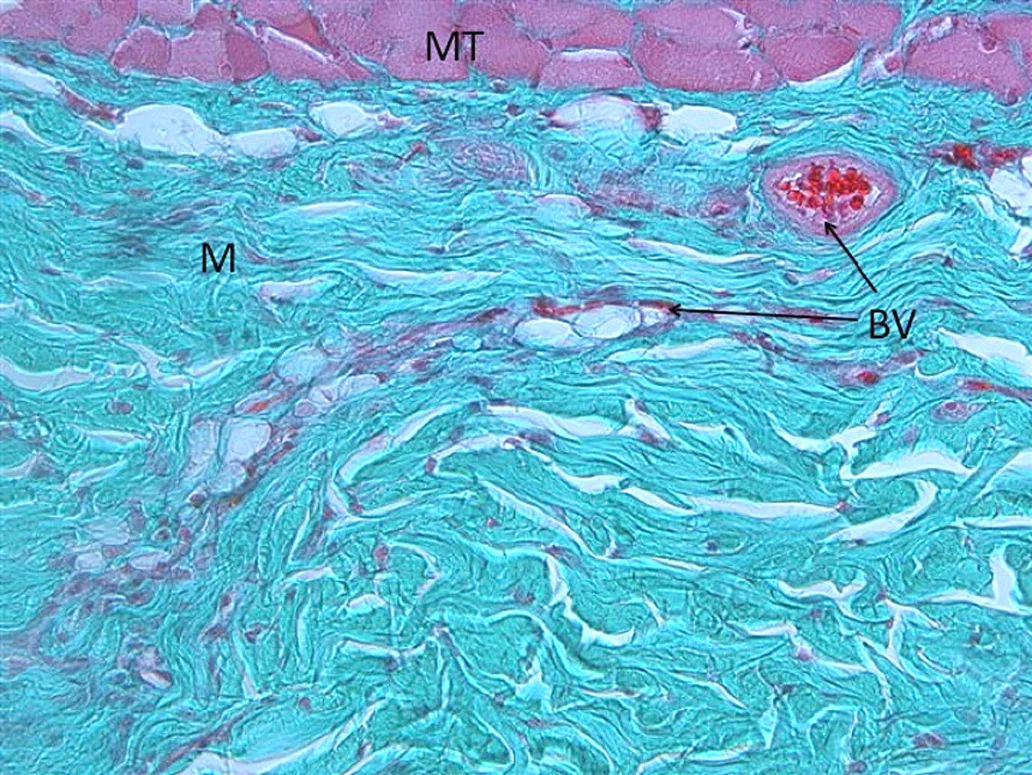
The first blood vessels (BV) are sprouting into Smartbrane (M), no signs of inflammatory reactions.
Scientific literature & clinical studies.
| 56 EUR | 0121.200 | SMARTBRANE membrane | 10 x 10 mm |
| 93 EUR | 0121.201 | SMARTBRANE membrane | 15 x 20 mm |
| 113 EUR | 0121.202 | SMARTBRANE membrane | 20 x 30 mm |
| 179 EUR | 0121.203 | SMARTBRANE membrane | 30 x 40 mm |
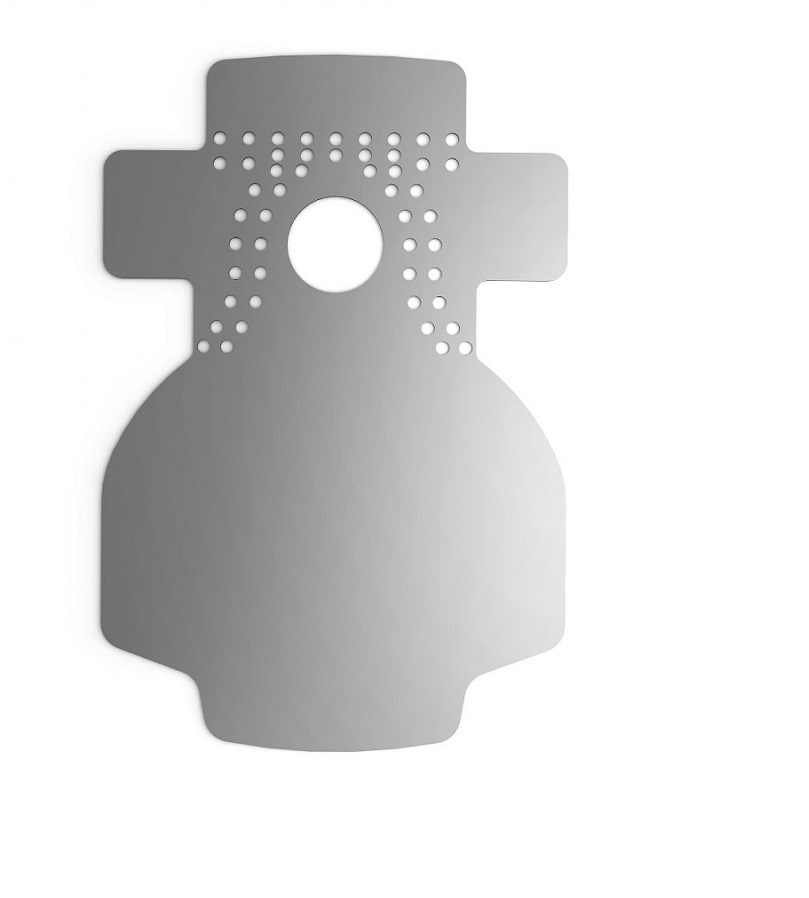
Titanium-Foil is ideal for threedimensional bone regeneration (GBR, Guided Bone Regeneration).
If necessary, it can be attached with pins or screws.
Titanium is a safe material with an excellent history in all the surgical procedures.
The fully impermeable Titanium-Foil is prestressable, stable and acts as a space maker, e.g. for alveolar ridge augmentation. Surgitime Titanium SEAL neutral bioelectrically thanks to electrochemical passivation and thus contribute to an uneventful growth of new bone.
Surgitime Titanium Seal is very flexible and can be used to cover periodontal or alveolar defects and generally does not requirefixation, but if necessary, the Bionnovation graft and fixation screw accessory can be used.
The Titanium Seal is indicated for alveolar sealing procedures, protecting the surgical wound against the invagination of the soft tissues, which promotes resorption of the alveolar process. Therefore, there is a statistically proven decrease of absorption reduction. It should be used through the modeling of the mesh with the careful covering of the operated zone completely with a margin that varies from 2 to 4 mm. It should not be reprocessed and attention must be paid to the sterilization period and correct instrumentations. Regarding the restrictions, the professional is responsible for choosing the implementation site, that is, he must carefully consider its use in the esthetic environment. Due to its color, it may cause some discomfort from the social point of view. Due to its malleability, it can be cut to adapt to the surgical sites and for being bio-electrically neutral thanks to the electrochemical passivation, it contributes to new bone growth without intercurrences.
It is recommended that the membrane stay in place for 21 days. This provides sufficient time for the initiation of osteoconduction and 14 days if used to seal a fresh extraction socket.
Increased tissue isolation.
Auto- fastener (not require fixing screws).
Total occlusivity.

Easy removal.
Used in post-extraction defects, and isolation or occlusion of sinus buco communications.
Extremely low biofilm retention.
| 76 EUR | 34x25 mm | 16890 |
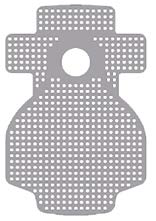
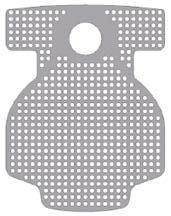
Surgitime Titanium is a nonabsorbable titanium screen made with pure Titanium (ASTM F-67) and has different sizes, thicknesses and hole diameters in order to meet the different clinical needs.
It is supplied sterile, as long as it is kept under ideal storage and preservation conditions and the integrity of the pack is not compromised. It is sterilized by Gamma Radiation (25kGy).
Surgitime Titanium aids in bone neoformation, acting as a barrier that prevents the migration of epithelial cells and connective tissue, avoiding the competition with the bone graft.
The titanium mesh provides excellent biocompatibility, occlusive property, its permeability enables the transmission of nutrients, easy use because it is very malleable and can be cut to adapt to surgical sites, has the capacity of keeping the regenerative space whole and enables vascularization of the graft on both sides (periosteum and endosteum). It was designed to ensure the three-dimensional reconstruction of alveolar bone defects and to facilitate bone replacement through suitable fixation of the replacement material.

Immobilization of the graft / membrane complex is a major factor for the success of bone reconstruction. It also depends on the correct selection of grafting biomaterials and the use of regenerative membranes. Care with the size and location of the flap, careful removal without damaging the periosteum and primary closure without tension, are fundamental for a good postoperative.
Surgitime Titanium should be shaped according to the anatomy of the bone and should not be bent at sharp angles, scratched or deformed. Once used and molded, it should not be molded again as it may result in product function failure.
The necessary stay for starting the osteoconduction is at least 21 days.
| 76 EUR | 34 x 25 mm, thickness: 0,04 mm, hole: 0,85 mm | 16472 |
| 76 EUR | 34 x 25 mm, thickness: 0,04 mm, hole: 0,15 mm | 16565 |
| 76 EUR | 34 x 25 mm, thickness: 0,08 mm, hole: 0,85 mm | 16698 |
Titanium material has an excellent biological stability, and it is beneficial to bone formation. Produced in titanium pure Gr 1 [ASTM F-67]. It is sterilized by Gamma Radiation [25kGy].
Due to its reduced thickness 0.08 mm, can be easily shaped into the desired shape.
It has a superior strength than absorbable membrane and excellent retention of space. High durability.
Customized for all degrees of bone defect is categorized as 3 different shapes:
Buccal / Proximal
medial – distal
2 wall
Buccal / Proximal
medial – distal
Lingual
3 wall

Buccal
1 wall
| 76 EUR | 3d F 12x18mm | 161256 |
| 76 EUR | 3d L 12x18mm | 161261 |
Surgitime PTFE is a nonabsorbable membrane composed of Polytetrafluoroethylene, with thickness
of 0.10 or 0.25 mm.
Surgitime PTFE is 100% biocompatible, synthetic and not of animal origin. It is indicated for in orthopedic, neural, maxillofacial procedures and other medical or dental surgery procedures. The polytetrafluoroethylene (PTFE) membranes or mechanical barriers for RTG (Guided Tissue Regeneration) has the aim of preventing the migration of epithelial and connective tissue cells, which would cause the inhibition of bone growth, promoting suitable space for the formation of a natural fibrin framework, the bone tissue precursor. The membrane avoids the tissue competition between the connective tissue and the bone, and has the purpose of isolating the bone grafts promoting tissue regeneration

| 45 EUR | 30 x 20 mm, thickness: 0,10 mm | 16021 |
| 45 EUR | 30 x 20 mm, thickness : 0,25 mm | 16044 |